Team I Genome Assembly Group: Difference between revisions
Lmckinney8 (talk | contribs) No edit summary |
Lmckinney8 (talk | contribs) No edit summary |
||
| Line 23: | Line 23: | ||
==== Team Goals ==== | ==== Team Goals ==== | ||
1. To perform quality control on reads before and after assembling the genome. | 1. To perform quality control on reads before and after assembling the genome. | ||
Before: | |||
** [https://github.com/OpenGene/fastp FASTp] | |||
After: | |||
** [http://bioinf.spbau.ru/quast QUAST] | |||
** [https://www.sanger.ac.uk/science/tools/reapr REAPR] | |||
2. To evaluate the performance of assembly tools: | 2. To evaluate the performance of assembly tools: | ||
** [https://www.bcgsc.ca/resources/software/abyss Abyss] | ** [https://www.bcgsc.ca/resources/software/abyss Abyss] | ||
** [https://galaxyproject.github.io/training-material/topics/assembly/tutorials/unicycler-assembly/tutorial.html Unicycler] | ** [https://galaxyproject.github.io/training-material/topics/assembly/tutorials/unicycler-assembly/tutorial.html Unicycler] | ||
| Line 33: | Line 40: | ||
** [https://www.psc.edu/user-resources/software/masurca MaSuRCA] | ** [https://www.psc.edu/user-resources/software/masurca MaSuRCA] | ||
** [https://www.ebi.ac.uk/~zerbino/velvet/ Velvet] | ** [https://www.ebi.ac.uk/~zerbino/velvet/ Velvet] | ||
3. To use the best tool to perform de novo assembly based on the 50 isolates. | 3. To use the best tool to perform de novo assembly based on the 50 isolates. | ||
Revision as of 16:40, 16 February 2020
Team 1 Genome Assembly
Team members: Lawrence McKinney, Laura Mora, Jessica Mulligan, Heather Patrick, Devishi Kesar, and Cecilia (Hyeonjeong) Cheon
Introduction
In bioinformatics, sequence assembly of a genome is the first of many steps involved to identify and characterize a potential pathogen. It is often considered the most important step in the stages of analysis and interpretation because of the challenge that still persists concerning high quality genome assembly [4]. Using the most relevant and high-quality tools are important for maintaining scientific rigor and more importantly, the results may have implications in public health that may affect many lives.
Stages of analysis and interpretation of data
1 - genome assembly 2 - gene prediction 3 - functional annotation 4 - comparative genomics 5 - production of a predictive webserver
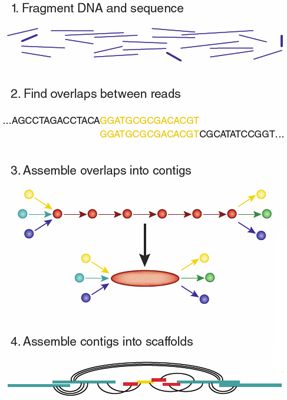
The basic principle of assembly principle of assembly is to note that the more similarity that exists between the end of one read and the beginning of another, the more likely they are to have originated from overlapping stretches of the genome. The output of an assembly is typically a set of ‘‘contigs,’’ which are contiguous sequence fragments, ordered and oriented into ‘‘scaffold’’ sequences, with gaps between contigs within scaffolds representing regions of uncertainty. There are numerous subclasses of assembly problems that can be distinguished by, among other things, the nature of: (1) the reads, (2) the types of sequences being assembled, and (3) The availability of homologous (related) and previously assembled sequences, such as a reference genome or the genome of a closely related species.
Figure 1. Genome Assembly Overview (https://www.nature.com/articles/nmeth.1935#citeas)
Team Goals
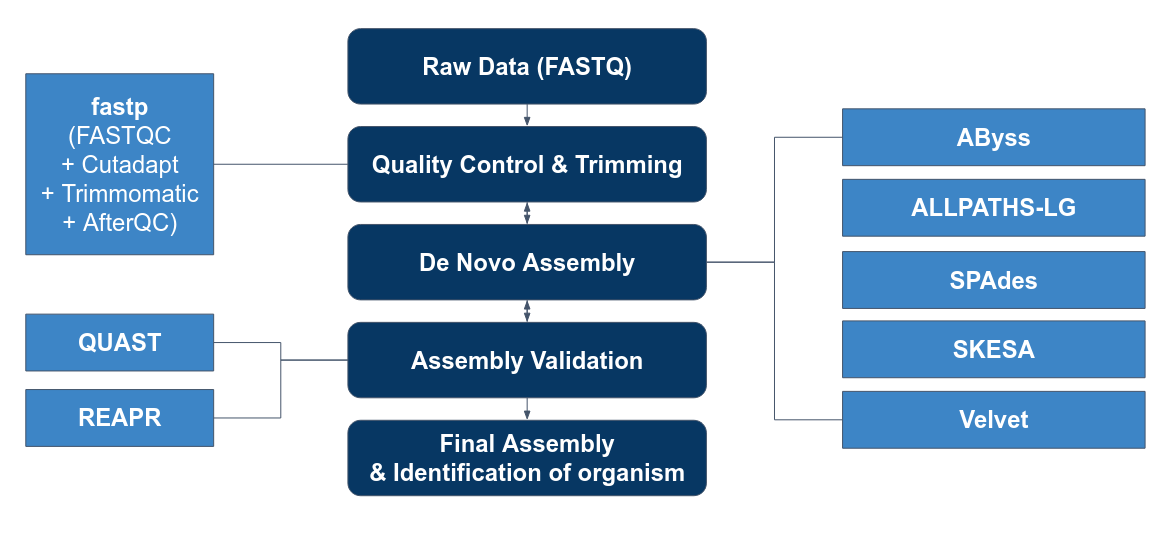
1. To perform quality control on reads before and after assembling the genome.
Before:
After:
2. To evaluate the performance of assembly tools:
3. To use the best tool to perform de novo assembly based on the 50 isolates.
4. To send off the highest quality result to the gene prediction team.
Methods
Genome Assembly Pipeline
Pre-processing
Trimming reads
Assembly
de Novo Assembly
Results
Conclusion
In-Class Presentations
File:Team 1 Genome Assembly Presentation 1.pdf
References
1. Alexey Gurevich, Vladislav Saveliev, Nikolay Vyahhi, Glenn Tesler, QUAST: quality assessment tool for genome assemblies, Bioinformatics, Volume 29, Issue 8, 15 April 2013, Pages 1072–1075, https://doi.org/10.1093/bioinformatics/btt086
2. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–477. doi:10.1089/cmb.2012.0021
3. Butler, Jonathan et al. “ALLPATHS: de novo assembly of whole-genome shotgun microreads.” Genome research vol. 18,5 (2008): 810-20. doi:10.1101/gr.7337908
4. Earl, Dent et al. “Assemblathon 1: a competitive assessment of de novo short read assembly methods.” Genome research vol. 21,12 (2011): 2224-41. doi:10.1101/gr.126599.111
5. Maccallum, Iain et al. “ALLPATHS 2: small genomes assembled accurately and with high continuity from short paired reads.” Genome biology vol. 10,10 (2009): R103. doi:10.1186/gb-2009-10-10-r103
6. Miller, Jason R et al. “Assembly algorithms for next-generation sequencing data.” Genomics vol. 95,6 (2010): 315-27. doi:10.1016/j.ygeno.2010.03.001
7. Pritt, J., Chen, N. & Langmead, B. FORGe: prioritizing variants for graph genomes. Genome Biol 19, 220 (2018). https://doi.org/10.1186/s13059-018-1595-x
8. Quainoo, S., Coolen, J.P., Hijum, S.A., Huynen, M.A., Melchers, W.J., Schaik, W.V., & Wertheim, H.F. (2017). Whole-Genome Sequencing of Bacterial Pathogens: the Future of Nosocomial Outbreak Analysis. Clinical microbiology reviews, 30 4, 1015-1063 .
9. Rahman, A., Pachter, L. CGAL: computing genome assembly likelihoods. Genome Biol 14, R8 (2013). https://doi.org/10.1186/gb-2013-14-1-r8
10. Salzberg, Steven L et al. “GAGE: A critical evaluation of genome assemblies and assembly algorithms.” Genome research vol. 22,3 (2012): 557-67. doi:10.1101/gr.131383.111
11. Shifu Chen, Yanqing Zhou, Yaru Chen, Jia Gu; fastp: an ultra-fast all-in-one FASTQ preprocessor, Bioinformatics, Volume 34, Issue 17, 1 September 2018, Pages i884–i890, https://doi.org/10.1093/bioinformatics/bty560
12. Sohn, Jang-il; Nam, Jin-Wu. “The present and future of de novo whole-genome assembly”, Briefings in Bioinformatics, Vol 19.1 (2018). doi.org/10.1093/bib/bbw096
13. Souvorov A., Agarwala R., & Lipman D.J. SKESA: strategic k-mer extension for scrupulous assemblies. Genome Biology. 2018; 19(1). doi:10.1186/s13059-018-1540-z
14. Tanja Magoc, Stephan Pabinger, Stefan Canzar, Xinyue Liu, Qi Su, Daniela Puiu, Luke J. Tallon, Steven L. Salzberg, GAGE-B: an evaluation of genome assemblers for bacterial organisms, Bioinformatics, Volume 29, Issue 14, 15 July 2013, Pages 1718–1725, https://doi.org/10.1093/bioinformatics/btt273
15. Zerbino, D., & Birney, E. (n.d.). Velvet: de novo assembly using very short reads. Hinxton: European Bioinformatics Institute.